Taxes, accounting, law and more. All the key news for your business.
Jiří Jakoubek | | March 9, 2023
We would like to inform you of the potential implications of a price regulation[1] of the of the ministry of healthcare, which came into force at the beginning of this year, in terms of its impact on transfer pricing.
The main objective of the price regulation is to regulate the price of medicinal products or foodstuffs intended for special medical purposes if they are covered by public health insurance, regardless of whether they are actually covered by public health insurance in the given case or or the patient pays for them in full. Price regulation is ensured in two ways, i.e. regulation of the originator price by setting a maximum price and regulation of the trade mark-up[2] by setting a maximum trade mark-up.
In terms of impact on the issue of transfer pricing, a fundamental change is the modified definition of the terms another person and originator price.
|
amendment effective until 31 December 2022 |
New regulation effective from 1 January 2023 |
|
“another person placing a registered medicinal product on the market (means) – a person authorised for distribution activities who places registered mass-produced medicinal products on the market in the Czech Republic, while not being in the position of the holder of the decision on their marketing authorisation or supplying them to the holder of the marketing authorisation.”
|
“another person supplying a medicinal product to the market (means) – a person authorised to carry out distribution activities who supplies mass-produced medicinal products to the market in the Czech Republic and forms a concern with the originator or is the only one to have been authorised in writing by the originator to supply such products to the market in the Czech Republic.” |
The initial amendment changes the very definition of another person to the person supplying the medicinal product to the market – not just placing the authorised product on the market, as was the case in the previous regulation. Thus, another person is defined by the cases of:
Therefore, the Pricing Regulation introduces a new requirement in relation to another person supplying the medicinal product to the market that this other person be part of a concern with the originator or be exclusively and in writing authorised by the originator.
A minor change has also been made to the definition of the originator price. In the new wording, it is the price, at which the medicinal product is supplied by the originator or another person supplying the medicinal product on the market in the Czech Republic to another person (it replaced the original wording: the price, at which it is supplied to the first person) authorised to distribute or dispense the medicinal product, excluding trade mark-up and VAT.
The new price regulation introduces a definition for the concept of price to the final consumer, which is defined as the sum of the price actually applied by the originator, the trade mark-up and VAT.
The above changes are summarised in the following simplified diagram.
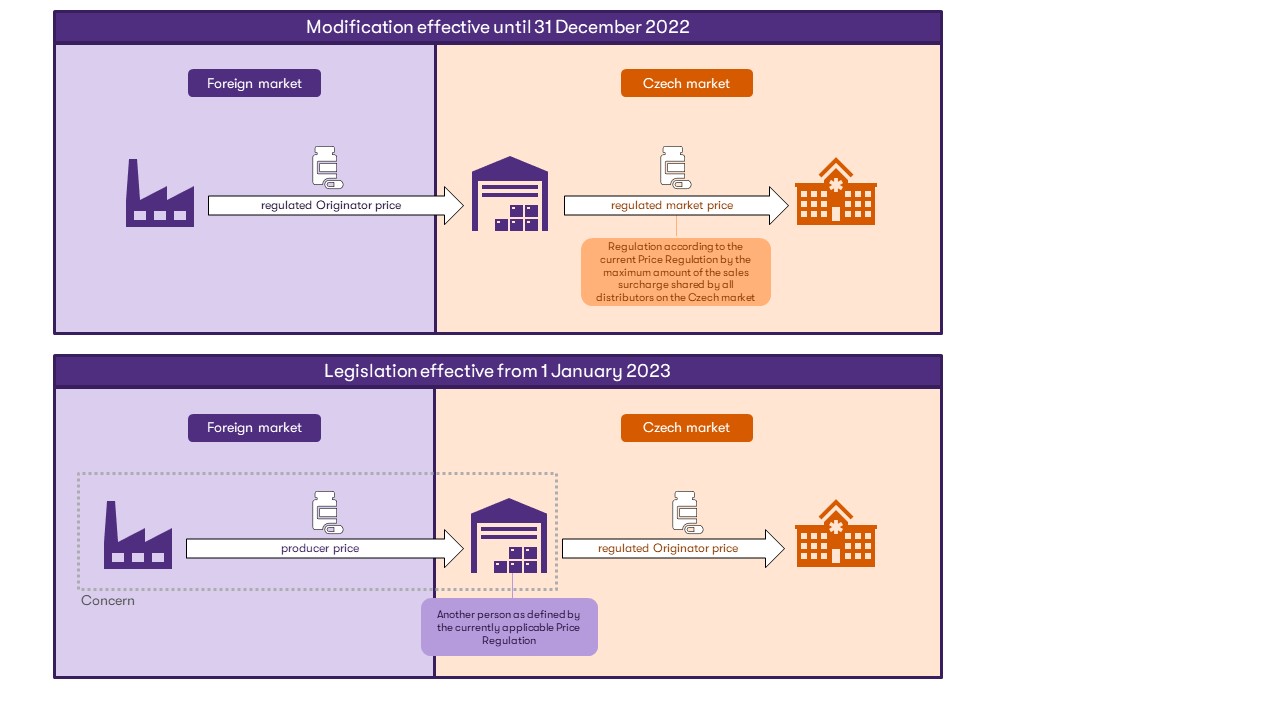
The above-mentioned change raises a number of questions not only in relation to the issue of transfer pricing.
The new price regulation may affect the existing pricing method between the originator and the distributor, which meets the conditions set by the currently valid Price Regulation for the definition of another person, because in this case the price, at which the medicinal product enters the Czech market, is regulated by the so-called originator’s price on the level of another person and at the same time should meet the tax rules related to the area of transfer prices.
If the above-mentioned impacts of the Price Regulation affect intra-group transactions carried out by your company, we will be happy to assist you in revising the current pricing model to comply with the arm’s length principle while respecting the rules imposed by the current Price Regulation.
[1] This is Ministry of Health Price Regulation No. 2/2023OLZP, on the regulation of prices of medicinal products and foodstuffs for special medical purposes, which replaces the existing Ministry of Foreign Affairs Price Regulation No. 1/2020/CAU of 10 December 2019 on the regulation of prices of medicinal products and foodstuffs for special medical purposes, which has been in force since January 2020.
[2] In the case of medicinal products and foodstuffs for special purposes specified in paragraph 7 of Article II. of the Price regulation (e.g. mass-produced registered radiopharmaceuticals), only the trade mark-up is subject to regulation.
[3] The originator for medicinal products is generally understood to mean the marketing authorisation holder, if the medicinal product is authorised, or the importer or domestic manufacturer, as appropriate.
Author: Jiří Jakoubek, Blanka Trefná